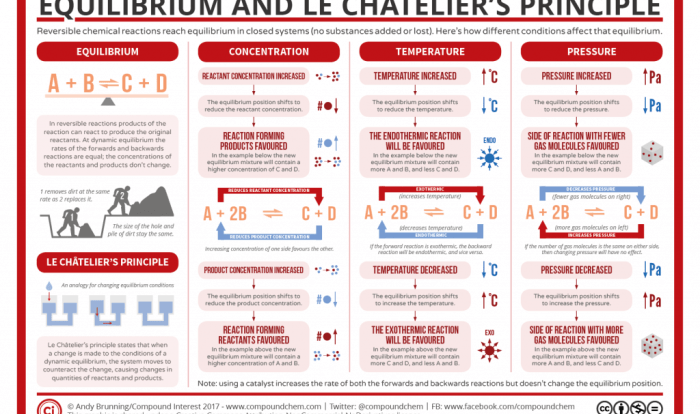
What dissolved species are present in a solution of KCN? This question delves into the intriguing world of chemistry, where we explore the behavior of substances when they interact with water. As we delve deeper into this topic, we will uncover the fascinating properties of KCN and the species it forms in aqueous solutions.
When KCN dissolves in water, it undergoes a process called ionization, where the molecules break apart into their constituent ions. This process is influenced by several factors, including temperature and the concentration of the solution. The resulting solution contains a mixture of dissolved species, each with its unique chemical properties.
Ionization of KCN in Water: What Dissolved Species Are Present In A Solution Of Kcn
When KCN dissolves in water, it undergoes ionization, a process in which the molecule dissociates into its constituent ions.
The chemical equation for the ionization of KCN in water is as follows:
KCN(aq) + H2O(l) → K+(aq) + OH-(aq) + HCN(aq)
The degree of ionization of KCN in water is affected by several factors, including the temperature, the concentration of KCN, and the presence of other ions in the solution.
Dissolved Species Present in KCN Solution
The dissolved species present in a solution of KCN include:
- K+ ions: These ions are formed when KCN dissociates in water.
- OH- ions: These ions are also formed when KCN dissociates in water.
- HCN molecules: These molecules are formed when KCN reacts with water.
- CN- ions: These ions are formed when HCN dissociates in water.
The concentration of each of these dissolved species is affected by the concentration of KCN in the solution.
Equilibria in KCN Solution
Several equilibria are established in a solution of KCN. These equilibria include:
- The ionization equilibrium: This equilibrium is established between KCN and its ions.
- The dissociation equilibrium: This equilibrium is established between HCN and its ions.
- The hydrolysis equilibrium: This equilibrium is established between KCN and water.
The equilibrium constants for these equilibria can be used to calculate the concentrations of the dissolved species in the solution.
Applications of KCN Solutions
KCN solutions are used in a variety of applications, including:
- Electroplating: KCN solutions are used to electroplate metals, such as gold and silver.
- Photography: KCN solutions are used to develop photographic film.
- Mining: KCN solutions are used to extract gold from ore.
The dissolved species in KCN solutions play an important role in these applications.
Commonly Asked Questions
What is the chemical equation for the ionization of KCN in water?
KCN(aq) + H2O(l) → K+(aq) + CN-(aq)
What are the factors that affect the degree of ionization of KCN in water?
Temperature and concentration
What are the applications of KCN solutions?
Electroplating, photography, and analytical chemistry