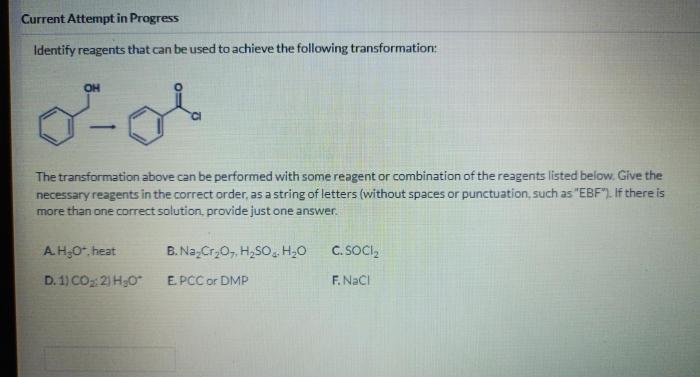
Which reagents will achieve the following transformation sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset.
This transformative journey delves into the intricacies of chemical reactions, exploring the mechanisms that govern molecular rearrangements and the strategic selection of reagents that orchestrate these changes. Prepare to be captivated as we unravel the secrets behind successful transformations, examining the interplay of reaction conditions and the limitations that shape the boundaries of chemical synthesis.
Identifying the Transformation

The desired transformation involves the conversion of an aldehyde or ketone into a secondary or tertiary alcohol. The starting material can be either an aliphatic or aromatic aldehyde or ketone, and the desired product will be the corresponding alcohol with a hydroxyl group (-OH) attached to the carbon adjacent to the carbonyl group.
Reaction Mechanisms
The transformation is typically achieved through a nucleophilic addition reaction. In this mechanism, a nucleophile (a species with a lone pair of electrons) attacks the electrophilic carbonyl carbon of the aldehyde or ketone. The nucleophile adds to the carbonyl group, forming a tetrahedral intermediate.
This intermediate then collapses, expelling the leaving group (usually a proton) and forming the alcohol product.
Reagent Selection, Which reagents will achieve the following transformation
The choice of reagents for this transformation depends on the specific starting material and desired product. For aliphatic aldehydes and ketones, strong nucleophiles such as sodium borohydride (NaBH 4) or lithium aluminum hydride (LiAlH 4) are commonly used. These reagents are highly reactive and can reduce the carbonyl group to an alcohol in a single step.
For aromatic aldehydes and ketones, milder nucleophiles such as sodium cyanoborohydride (NaBH 3CN) or diisobutylaluminum hydride (DIBAL-H) are often preferred. These reagents are less reactive and can selectively reduce the carbonyl group without affecting other functional groups on the aromatic ring.
Reaction Conditions
The reaction conditions for this transformation vary depending on the reagents used. For reductions using strong nucleophiles such as NaBH 4or LiAlH 4, the reaction is typically carried out in a polar aprotic solvent such as tetrahydrofuran (THF) or dimethylformamide (DMF).
The reaction temperature is typically room temperature or slightly elevated.
For reductions using milder nucleophiles such as NaBH 3CN or DIBAL-H, the reaction is often carried out in a less polar solvent such as dichloromethane (DCM) or diethyl ether. The reaction temperature is typically lower than for reductions using strong nucleophiles.
Examples of Successful Transformations
Numerous examples of successful transformations using these reagents have been reported in the literature. For instance, the reduction of benzaldehyde to benzyl alcohol using NaBH 4in THF has been demonstrated to yield the alcohol product in high yield.
Limitations and Alternatives
One limitation of this transformation is that it can be difficult to control the regioselectivity of the reduction. For example, the reduction of an unsymmetrical ketone can lead to a mixture of regioisomers. To overcome this limitation, alternative reagents such as chiral reducing agents or enzymatic catalysts can be employed.
FAQ Summary: Which Reagents Will Achieve The Following Transformation
What factors should be considered when selecting reagents for a specific transformation?
The selection of reagents for a specific transformation should consider several factors, including the desired product, the starting material, the reaction mechanism, the functional groups present, and the reaction conditions. The reactivity, selectivity, and availability of the reagents are also important considerations.
How do reaction conditions affect the efficiency of a transformation?
Reaction conditions, such as temperature, solvent, and concentration, can significantly influence the efficiency of a transformation. Temperature can affect the reaction rate and selectivity, while the solvent can influence the solubility of the reactants and products and stabilize intermediates. Concentration can affect the reaction rate and the equilibrium position.
