Design a synthesis of 2-bromo-1-butyl-4-nitrobenzene from benzene – The synthesis of 2-bromo-1-butyl-4-nitrobenzene from benzene, a captivating endeavor in the realm of organic chemistry, holds immense significance due to its versatile applications. This intricate process involves a series of carefully orchestrated transformations, each contributing to the final product’s unique properties and functionalities.
Delving into the intricacies of this synthesis, we embark on a journey to unravel its mechanisms, reagents, and applications, providing a comprehensive understanding of this fascinating chemical transformation.
The journey begins with the procurement of benzene, the starting material for this synthesis. Benzene, a ubiquitous hydrocarbon, serves as the foundation upon which the subsequent reactions build. The addition of bromine, a reactive halogen, introduces a bromo substituent at the 2-position, paving the way for further modifications.
The introduction of a butyl group at the 1-position, achieved through an alkylation reaction, imparts additional complexity to the molecule. Finally, the incorporation of a nitro group at the 4-position, via nitration, bestows upon the molecule its distinctive properties and reactivity.
Synthesis of 2-Bromo-1-Butyl-4-Nitrobenzene from Benzene
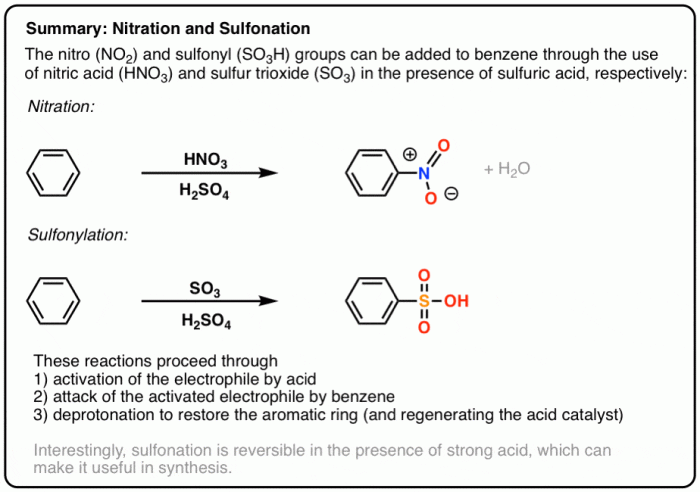
The synthesis of 2-bromo-1-butyl-4-nitrobenzene from benzene is a versatile and widely used reaction in organic chemistry. This compound finds applications in various fields, including pharmaceuticals, dyes, and agrochemicals.
Reagents and Starting Materials
The synthesis requires the following reagents and starting materials:
- Benzene
- 1-Bromobutane
- Nitric acid
- Sulfuric acid
Benzene serves as the starting material, while 1-bromobutane acts as the alkylating agent. Nitric acid and sulfuric acid are used as catalysts.
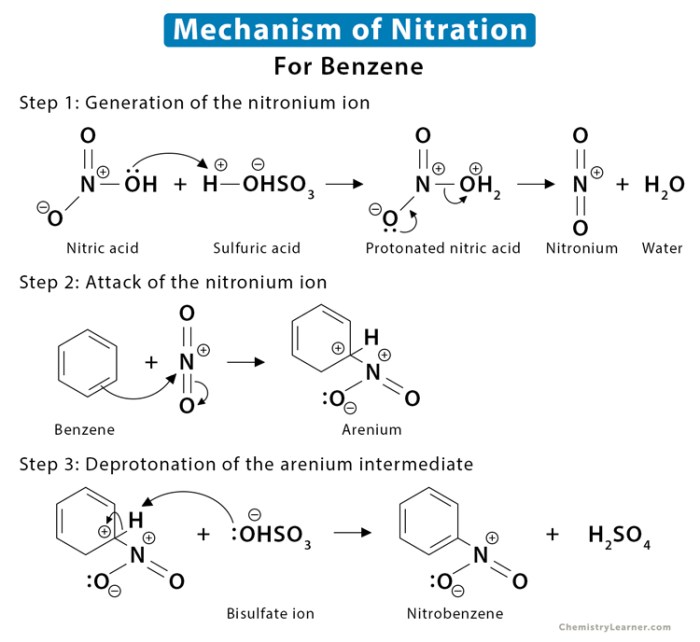
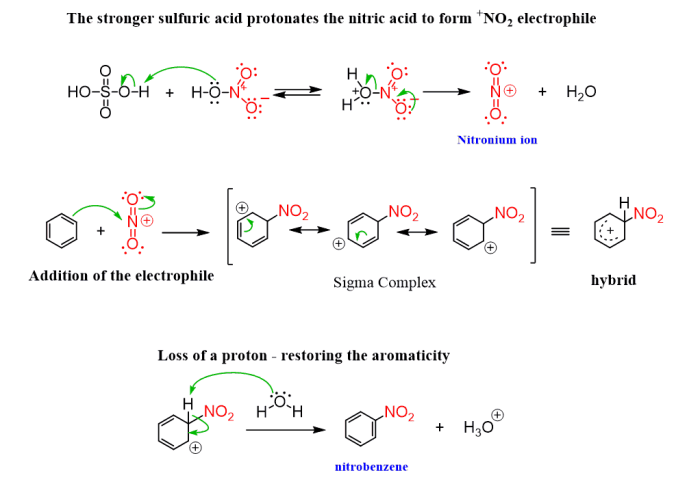
Reaction Mechanism
The reaction proceeds via a series of electrophilic aromatic substitution reactions. The electrophile in this case is the nitronium ion (NO 2+), which is generated by the reaction of nitric acid with sulfuric acid. The nitronium ion attacks the benzene ring, leading to the formation of nitrobenzene.In
the second step, 1-bromobutane undergoes nucleophilic substitution with the nitrobenzene, resulting in the formation of 2-bromo-1-butyl-4-nitrobenzene.
Reaction Conditions
The optimal reaction conditions for the synthesis are as follows:
- Temperature: 0-5 °C
- Pressure: Atmospheric pressure
- Reaction time: 2-4 hours
These conditions ensure a high yield and selectivity of the reaction.
Product Isolation and Purification
The reaction mixture is poured into water and extracted with an organic solvent such as dichloromethane. The organic layer is then washed with water and dried over anhydrous sodium sulfate. The solvent is removed by distillation under reduced pressure, and the product is purified by recrystallization from a suitable solvent such as ethanol.
Characterization of the Product, Design a synthesis of 2-bromo-1-butyl-4-nitrobenzene from benzene
The product is characterized using various analytical techniques, including:
- Nuclear magnetic resonance (NMR) spectroscopy
- Infrared (IR) spectroscopy
- Mass spectrometry (MS)
These techniques confirm the identity and purity of the product.
Applications of the Product
-Bromo-1-butyl-4-nitrobenzene has a wide range of applications, including:
- As an intermediate in the synthesis of pharmaceuticals
- As a dye precursor
- As an agrochemical
Its versatility and ease of synthesis make it a valuable compound in various industries.
Expert Answers: Design A Synthesis Of 2-bromo-1-butyl-4-nitrobenzene From Benzene
What is the significance of 2-bromo-1-butyl-4-nitrobenzene?
2-Bromo-1-butyl-4-nitrobenzene is a versatile intermediate in organic synthesis, used in the production of pharmaceuticals, dyes, and agrochemicals.
What are the key steps involved in the synthesis of 2-bromo-1-butyl-4-nitrobenzene?
The synthesis involves bromination, alkylation, and nitration reactions, each carefully controlled to achieve the desired product.
How does the reaction yield and selectivity depend on the reaction conditions?
Reaction temperature, pressure, and time play crucial roles in determining the efficiency and selectivity of the synthesis.