Chemical equilibrium and Le Chatelier’s principle lab report unveils the fundamental concepts of chemical equilibrium and its dynamic interplay with external factors. This report delves into the theoretical underpinnings of chemical equilibrium, exploring its significance in various scientific disciplines and showcasing the practical applications of Le Chatelier’s principle in predicting the direction of reactions.
The experimental component of this report meticulously Artikels a hands-on investigation designed to demonstrate the profound influence of concentration, temperature, and pressure on a chemical equilibrium system. Detailed instructions guide the reader through the experimental setup, providing a clear understanding of the materials and equipment required, as well as a step-by-step procedure for conducting the experiment.
Chemical Equilibrium and Le Chatelier’s Principle: Chemical Equilibrium And Le Chatelier’s Principle Lab Report
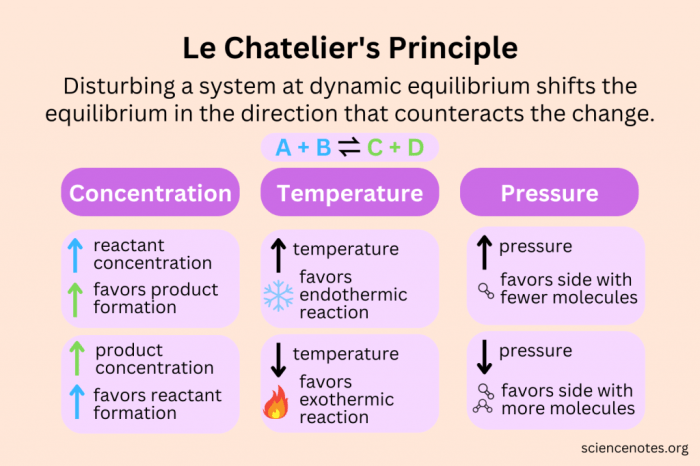
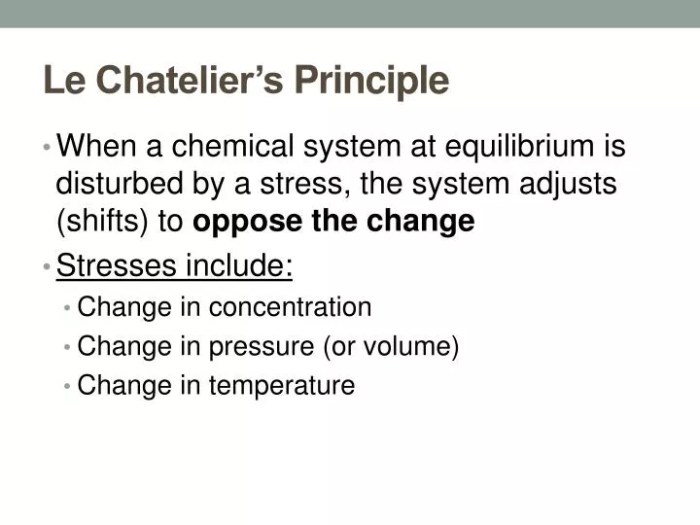
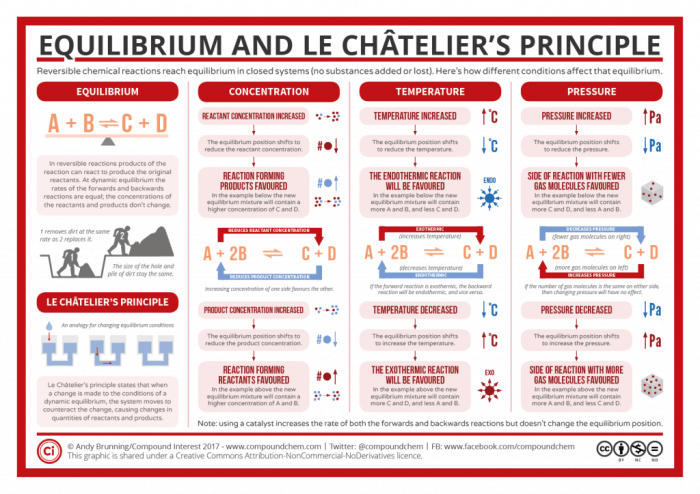
Chemical equilibrium is a dynamic state in which the concentrations of reactants and products in a chemical reaction remain constant over time. Le Chatelier’s principle is a useful tool for predicting the direction of a reaction when the conditions are changed.
Importance of Chemical Equilibrium
- Predicting the behavior of chemical systems in various fields, such as industrial chemistry, environmental science, and biochemistry.
- Designing processes for synthesizing chemicals, controlling pollution, and understanding biological systems.
Applications of Le Chatelier’s Principle
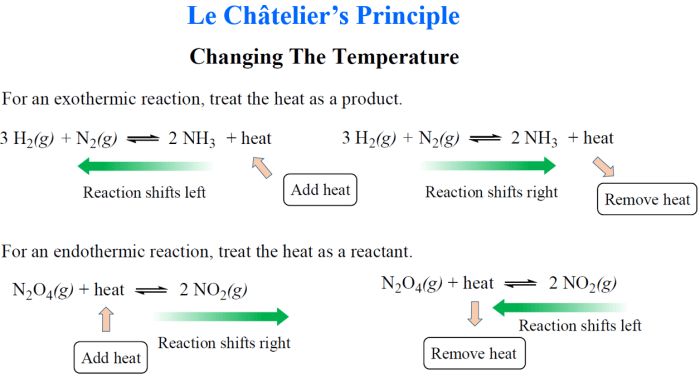
- Predicting the direction of reactions by considering the effects of changes in concentration, temperature, and pressure.
- Optimizing chemical processes by manipulating conditions to favor desired products.
Experimental Setup, Chemical equilibrium and le chatelier’s principle lab report
An experiment can be designed to demonstrate the effect of changing concentration, temperature, and pressure on a chemical equilibrium system, such as the reaction between hydrogen and iodine to form hydrogen iodide.
Materials and Equipment:
- Hydrogen gas
- Iodine crystals
- Sealed glass container
- Thermometer
- Pressure gauge
Procedure:
- Place a small amount of iodine crystals in a sealed glass container.
- Introduce hydrogen gas into the container and measure the initial pressure.
- Monitor the temperature and pressure as the reaction proceeds.
- Repeat the experiment with different initial concentrations of hydrogen and iodine, or at different temperatures and pressures.
Question Bank
What is the significance of chemical equilibrium in the field of chemistry?
Chemical equilibrium plays a pivotal role in understanding the behavior of chemical reactions and predicting the distribution of reactants and products at a given set of conditions. It is essential for comprehending the dynamics of chemical systems in various disciplines, including biochemistry, environmental chemistry, and industrial chemistry.
How does Le Chatelier’s principle aid in predicting the direction of reactions?
Le Chatelier’s principle provides a valuable tool for predicting the direction of reactions by analyzing the impact of external factors such as concentration, temperature, and pressure on a chemical equilibrium system. By manipulating these factors, it is possible to shift the equilibrium position and control the formation of desired products.
What are the limitations of Le Chatelier’s principle?
Le Chatelier’s principle is a qualitative tool that provides general predictions about the direction of reactions. However, it does not provide quantitative information about the extent of the shift in equilibrium. Additionally, it is not applicable to reactions that involve multiple equilibria or complex reaction mechanisms.